Cruising sailors have long had a love-hate relationship with their onboard electronics for one simple reason: Water and electricity don’t mix. Water with dissolved salts forms a wonderful electrolyte. Boats are full of dissimilar metals, and the electrical currents generated in this electrolytic soup will oxidize contacts, increase resistance, and sometimes destroy components entirely.
The salts themselves sometimes join or initiate destructive reactions on their own; stainless steel in the absence of oxygen is prone to crevice corrosion; copper is prone to cracking at elevated temperatures; aluminum is extremely prone to pitting in the presence of most dissimilar metals; and tin is sacrificial in the presence of copper. In fact, none of the metals used in marine wiring are completely immune. Sound wiring methods, good materials, and dry locations all help, but on the older boat, we often confront legacy systems and problems, and we seek solutions short of complete reconstruction. Is there hope?

The local chandlery boasts shelves full of products that promise to displace moisture, lubricate, and prevent corrosion. In our experience, the effectiveness of these elixirs, at least in damp electrical system applications, has been disappointing. Do the protectants wash off? Evaporate? Are their reactive or effective components somehow consumed? These are all questions that have hung over us for years.
In our two-part test of electrolytic corrosion sprays (April and September 2007), we found six products that stood out-Boeshield T-9, Corrosion Block, Corrosion Pro Lube, CorrosionX, CRC Heavy Duty Corrosion Inhibitor, and TC-11. Since then, our attention has shifted to the wires and connections themselves, as well as some industrial protectants that we did not previously test.
The problem that reignited our interest in wire corrosion was a routine electrical failure. A poor spade connection on a shower sump pump gave way. Had this occurred aboard any other boat, the connectors would have been replaced and showers would be worry-free once more-until the connection failed again. But this was PS contributor Drew Fryes 32-foot 1997 PDQ Altair. Frye, an industrial chemist by trade, could not resist the temptation to learn more about the causes of the failure and how to prevent it from happening again. Shower time could wait.
It is commonly held that pre-tinned wires, high-quality connectors, and an anti-corrosion treatment will help prevent such failures, but to what degree? And which investment in time and energy yields the best results? Frye sought to answer these and other questions with an accelerated wire corrosion test. Shower time could wait . . . just not too long.
Test goals
Ultimately, the goal of this ongoing project is to examine corrosion in wires and connectors. More concisely, it could be presented as a closer look at three common elements in marine wiring:
Best wire for the job. Is it tinned wire, automotive wire, or stranded machine tool wire? We have not tested extension cord wire or other low-grade wires. The insulation on these wires is far inferior, and they have no place on a boat.
Best protectants. In industrial usage, there are several corrosion preventatives that are used in damp environments or places where aluminum and copper must meet. Noalox is one common product; contact grease is another. In refinery applications, Frye has been very pleased with the performance a cosmolene-type contact grease, No-Ox-Id.
Best connections. Electrolysis can occur both between the connector and wire (copper/tin) and between the crimp connector and the terminal (tin/copper, tin/aluminum). Where did it occur, and which samples were most prone to electrolysis?
Clearly, these questions generated many permutations. Terminal strips make evaluation and rating fairly systematic; but the data is better presented in the tables on these pages. As you look at the tables and results, keep in mind that the ranking scores are only within each group and do not cross-correlate.
Limits of Accelerated Testing
In his work as a chemist, Frye is familiar with many test methods for accelerated corrosion and product testing. Primarily, his work in this area has to do with engine coolants, cooling system corrosion, and long-term corrosion protection. Three lessons he learned through his work are continuously reinforced.

When performing accelerated testing, you should not exaggerate the test conditions to an excessive degree. For example, using oxygen to accelerate the breakdown of engine coolants results in failure modes that don’t actually occur. New chemical reactions take place, beyond those that actually occur in service.
The same is true of overly exaggerated temperature extremes, ultrasonic vibration, or increased pressures. The test conditions must be selected such that they do not cause failure types that do not occur in practice. In these tests, PS wanted to see the corrosion types and failures that we actually see on boats. This meant we did not want to use temperatures high enough to melt any of the corrosion-resistant compounds we will be studying, or add chemicals to the water that may react in ways we don’t understand. We can carefully increase the temperature, salinity, and exaggerate wetting and drying cycles, to severely exaggerate the conditions we would see in the bilge of a boat in a warm climate, without introducing any new variables. (See “Test Details,” above.)
In the marine world, electrolysis and galvanic corrosion are more serious problems than general corrosion. However, the damage caused by stray-current corrosion can be unstoppable and is beyond the scope of our test. For this project, Frye explored only galvanic corrosion, and he limited the metal pairings to those that are realistic.
In engine coolant testing, metals are exposed in groupings that are commonly found in engines: cast-iron/steel/aluminum and copper/solder/brass samples are assembled as groups with conductive steel spacers. However, the brass is not coupled to aluminum, because that is not done in engines.
In a similar approach, Frye only studied wire attached to tinned crimp connectors that are subsequently fastened to tinned copper terminal blocks, because this is the typical situation. He did not study tinned connectors on cheap plated terminal blocks because the result is foregone: They will fall apart under the test conditions. Aluminum and stainless steel combinations were also included, because, although rare, this pairing does occur on occasion.
How We Tested
Corrosion is a complex phenomenon, and proper treatment is far beyond the scope of this article; however, a brief overview might help explain the test methodology and why certain approaches work better than others.
For the purposes of this test, Frye wanted to create the possibility of failure. Top-quality shrink-fit connectors that are kept dry should be very reliable, and inducing failures in such perfect conditions is unlikely. He sought to make the conditions challenging, but not unrealistic.
Only a few sealed connectors were used, and that is because they are so effective. Push-on connections and terminal strip connections-more common points of failure-were used in greater numbers.
We tested three wire types: corrosion-resistant tinned marine wire, automotive primary wire, and thermoplastic high heat-resistant nylon-coated (THHN) machine tool wire (typically used in factories and chemical plants in conduit, but also sold at every hardware store). These do meet the appropriate codes, are less expensive and more available, and are perhaps more likely to fail. It is a test, and a test should be designed to encourage some failures. “Test Details” on the facing page explains the environmental chamber, and the section below subtitled “Evaluation of the Samples” explains the rating process. However, before getting into that, the materials themselves deserve a closer look.
Wires
For system conductors, the American Yacht and Boat Council (ABYC) recommends wire that meets Underwriter Laboratories UL1426, and Society of Automotive Engineers SAE J378 standards. Battery cables, low-tension primary cables have separate standards, and don’t concern this study.
Marine system wires are finely stranded and use high-quality insulation that is impervious to water, oil, and is resistant to oxygen permeation.
The Coast Guard approves the above, plus machine tool wire for all applications not directly connected to vibration sources such as motors. The National Electrical Code (NEC) also approves the above wires, plus machine tool wire for all applications not directly connected to vibration sources such as motors. Machine tool wire is of particular interest because its slightly thicker strands might hold up better to corrosion than the thinner strands of non-tinned automotive wire or tinned marine wire.
Automotive primary wire is about a half-gauge lighter than marine wire or machine tool wire, by code. All three wire types were tested; tinned wire made by Ancor, and automotive primary wire and machine tool wire from Southwire.
While most boaters are familiar with automotive wire or tinned, marine-grade wire, industrial machine tool wire deserves some explanation. In a chemical plant environment, the short pigtail of wire coming out of the electric motor will be tinned or non-tinned, finely stranded wire, much like marine wire. This is to withstand the high-frequency vibration of the motor.

Typically, connections are made using wire nuts, with or without corrosion preventative treatments. If exposure is expected, the connection is sealed in a water- and vapor-tight box mounted to the motor. From there and all through the conduit in the plant, stranded machine tool wire is used.
Instead of having the 26 strands, common in 16-gauge automotive and marine wire, industrial machine tool wire has 19 slightly thicker strands. The insulation is very high grade and will withstand abrasion, vibration, heat, and water for extremely long periods of time. Thus, this wire seems completely suitable for marine application except where tinned wire is needed in very damp locations and where high-frequency vibration is found in the engine room. It is approved by the NEC and Coast Guard for these applications.
Some boaters have reported that machine tool wire holds up better because the thicker strands are not as severely damaged by corrosion. Although connections made with machine tool wire would be expected to fail at the same rate as connections of other types, the entire wire might not be ruined as quickly, because the thicker strands would better resist corrosion that tends to spread up the wire beneath the insulation. This is an interesting avenue to explore, and one we hope to answer at the end of this experiment. The value of the tin coating on marine wire versus plain copper primary wire is also of interest.
Connections
We did not test wire nuts as they do not meet any marine code. We also did not test solder-only connections, tenuous for typical marine applications. We tested only well-formed crimps made with a ratchet crimper. These meet the industry standard, are easy to make, and have proven very reliable. (See “Tech Tips.”)
The clamping force in a good crimper is so great that the copper and tin components of the fitting and wire flow together and leave no space for air or water to intrude, and no place for corrosion to begin. All fittings for this test were made by Ancor (ring crimp connectors and terminal blocks) or Ideal (heat-sealed crimp connectors and push-on connectors). All were tinned copper.
Spade connectors.
Terminal strips.
Aluminum terminal strips. Though aluminum is not an accepted terminal material, a significant number of older boats use the mast as a ground conductor for lighting circuits, so we also included an aluminum strip. Maintaining a good contact at the mast can be a significant challenge, since the ground conductor-mast connection is often in a very wet area. Faults on the mast may not be difficult to troubleshoot, but they can be difficult to reach.
Corrosion Preventatives
Corrosion preventatives fall into several categories, but the three most common are what are most relevant here:
Spray-on and other thin-film products (Boeshield, Corrosion Block);
Thick greases (terminal greases like No-Ox-Id, and bearing grease);
Thick greases with sacrificial metal or other chemical components (Noalox).
All but one of the tested products can be defined as dielectric. (Noalox, which contains aluminum powder, is weakly conductive.) Dielectric compounds are, by design, excellent insulators, and it is this characteristic that gives them anti-corrosive properties. They block the flow of oxygen, water, and most importantly, electrons. Without an electric current, there can be no galvanic corrosion.
There are many off-the-shelf dielectric compounds that claim long-term protection. Some sailors swear by petroleum grease (Vaseline), and it is at least reasonably effective. The scope of this project, however, required a limited field.
We included five products, two that are commonly used in Fryes industry (Noalox and No-Ox-Id) and three that represent a good cross-section of whats available on the market (Corrosion Block, Boeshield T-9, and waterproof Green Grease bearing grease).
These products can be used both inside the crimp connection and around the terminal block. There has been a long-running debate as to whether grease will reduce conductivity in the connections or contacts. Generally, in high-pressure connections (crimps and bolted connections) a non-conductive product wont interfere, but thats because its squeezed out of the joint, which calls into question the utility of applying it in the first place. In low pressure connections (electronics only), however, there is potential for trouble.
Although our test did not concentrate on this topic, we were able to reach some general conclusions on the proper use of these materials.
Evaluation of the Samples
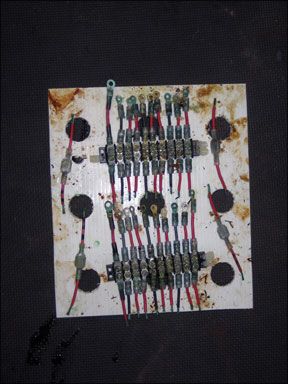
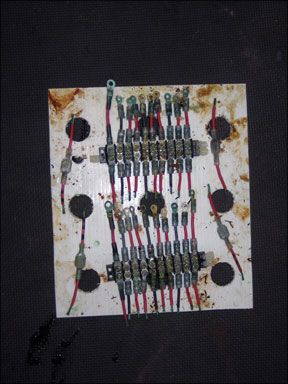
A visual inspection is a key part of the evaluation. We made observations during the test period without disturbing the samples. At the end of the full one-year test period, we will unbolt all of the fittings from the terminal strips and look for corrosion under the fittings. We will strip the wires and look for damage up the wire. This may be where tinned wire shines.
Continuity.
Current varied from 8.0 to 8.3 amperes, and the voltage varied from 13.2-13.3. The wiring used heavy jumper cables connected to the battery terminals, 12-gauge wire for small lines, and alligator clips to connect each sample in turn. It was necessary to wiggle the clamps through the surface corrosion on the wire ends in every case to get stable readings, but in all cases, a few wiggles were enough.
Resistance was determined by measuring the voltage difference across the test samples and applying Ohms Law: Ohms = volts/amperage.
We reported 0.05 ohms as the minimum value, as voltage differences below 0.4 volts were not sufficiently repeatable to report due to corrosion on the wire ends. In no case, other than two open circuit failures, did we find the lamp to be noticeably dim, a simple double check for our measurements. It is believed that true failures will show even greater increases in resistance over time.
Although 0.05 ohms sounds like a good connection, it does represent about 3 percent of the total circuit resistance. The worst connection (one of the aluminum strip samples) measured voltage drops of about 0.4 volts. At 8 amperes, this means the connection was generating 5 watts of heat, and a whole terminal strip would generate 50 watts of heat. A resistance of 1 ohm in an 8-ampere circuit will drop the current to 5-amps and make the light quite dim, since it will be operating on only 8 volts! So, it is easy to test the resistance of a high load circuit and get a “good” reading, and still have a dead circuit when placed under load.
Conclusions
Because the materials investigated are inherently corrosion resistant, we knew we were in for a lengthy project. There was no expectation of clearly observable changes in a few days or weeks, and yet a few things became clear from the start:
Two weeks
At this stage, long-term effects are already quite evident:
Tin is clearly protecting the copper by becoming a sacrificial anode. Some loss of tin from crimp connectors was apparent on unprotected samples as deposits appeared under the samples. It was clear that in the presence of uncontrolled galvanic corrosion, tin plating provides short-term protection.
Bare copper wire begins to darken unless protected with a coating or a sacrificial layer.
The thinnest of the anti-corrosion coatings began to fail almost immediately, as evidenced by darkening of the wire under the coatings.
It is clear that tinned wire will have the edge in repairability. While copper wire will be corroded under the insulation and thus difficult to re-crimp even after trimming back, tinned wire should be in better shape under the insulation.
Three months
At this stage, even high-quality wires begin to show their age.
The tin is fading away. It protects by being sacrificial, and only the most effective treatments are able to maintain good-looking ring terminals.
All of the wire ends are looking poor; however, the tinned wire was still less corroded.
Six months
After six months, we began continuity testing:
Surprisingly, as ugly as the wires and connections had become visually-and nearly all of them looked very bad-only two failures were observed. No other sample showed noticeable increases in resistance.
All of the bolted terminal connections showed less than 0.05 ohms resistance. Only the untreated push-on connectors had failed-both of the samples in the group. We pulled them apart, re-connected them, and they failed again. So, already we have proof that if you use this type of connection, a corrosion-preventative product is needed. Moreover, there are many such preventatives that are effective.
In all cases, the excess protection washed off the union, but there was still enough protective material trapped in the joint. A minimal amount will serve well.
General Observations
There are also some more general conclusions, observations, and extrapolations worth listing:
Push-on connectors are unreliable in damp environments, even if occasional equipment replacement or service is foreseen. Better, leave a coil of extra wire and use crimp connections. Alternatively, add a junction box in a drier location.
Heavy grease products work best, and sprays works only where little moisture is expected. Perhaps sprays can handle condensation-Frye has had success using them to protect metals in condensation prone enclosures-they cannot resist being washed off in tough locations.
Noalox (aluminum wiring corrosion preventative) and No-Ox-Id (terminal grease), the only grease products intended for this sort of use, out performed all others. However, because of the sacrifical nature of Noalox, it may have a shorter lifespan.
Push-on connectors in damp locations should always be filled with a corrosion preventative. This is good practice in all boat applications. Lubrication also greatly reduces the chance of seizing.
Aluminum is not suitable as a terminal material in damp marine environments. Though none of the samples failed in six months, within a year or so it seems clear some of them will simply fall apart.
There is no need to rip out all of the non-tinned wiring in a boat if there are no serious concerns with chafe or insulation deterioration. The problems with connections are not the fault of the wire but are more likely the result of poor crimp connections. If poor crimps are present, they may fail in damp environments.
Tinned wire does offer superior corrosion protection compared to non-tinned wire in damp environments and is a best-practice throughout the boat. In dry locations, tinned marine wire, primary wire, or machine tool wire are all acceptable and appropriate.
Tinned wire does not last forever in very poor conditions. The tin is a sacrificial coating, like galvanizing, and will be consumed if there are significant galvanic currents.
The most important factor in connection reliability is achieving a good, tight connection. That we produced no crimp failures speaks volumes for a good, tight crimp, where the parts are cold welded together.
Sample crimps should be tested. Pull them apart; the wire or the fitting should fail before the crimp. Cut a sample crimp in half with a Dremel tool and examine with a magnifying glass; there should be no visible boundaries between wire strands or between the wires and the fitting.
We found that when using low-pressure hand crimpers, we could still see voids in the crimp; with a properly adjusted ratchet crimper the metals flowed together.
While heat-shrink insulation adds reliability to any connection and prevents salt from creeping up inside the insulation and damaging the wire over time, there seems to be no benefit in dry locations; connections without heat shrink tubing successfully endured our torture chamber.
Heat-sealed butt connectors are required in locations subject to spray or immersion; it is the only practical means to seal the wire completely from the surrounding water and electrolyte, and to keep the electricity inside the wire. Neither terminal strips nor push-on connectors should be used in wet locations, because they cannot be sealed. Stray currents are both dangerous and very corrosive.
Sample exposure will be continued for an additional six months (1 year total), at which time they will be re-tested for resistance and disassembled for visual examination.
Best Anti-corrosion Coating
Noalox (aluminum wiring corrosion preventative) and No-Ox-Id (terminal grease), out performed all others by a wide range. Waterproof bearing grease and sprays could not resist being washed off, or failed to protect against corrosion. We found grease products simpler to use since there is no over-spray concern. At the same time, it should be apparent from the photographs that no product succeeded in protecting the exposed wire ends, whether copper wire or tinned copper wire.
The best value? Until long term testing is complete, this is still too close to call. Noalox is widely available from Home Depot, Lowes, and all electrical supply houses. No-Ox-Id or equivalent terminal grease products are available at auto parts stores and electrical supply houses.
The Best Wire Type
What about that expensive marine wire? If it is a damp location, spend the money. Though it probably has little bearing on the durability of the crimp or bolted connection, it will have a major bearing on the repairability and durability of the wire in severe applications. In dry location, it is your choice, as the code reflects.
Machine tool wire will be fine except where connected to extreme vibration sources. The toughest corrosion test is drying salt. If your wiring gets wet during a knock-down or similar event and then dries encrusted with salt, you will be much better off with tinned wire.
Frye did some work at a refinery in New Orleans that was flooded after Hurricane Katrina. He noted that the damage that began to appear later, as conduits that went under water began to fail from the effects of drying salt. He learned that the best thing for wiring that went under salt was either to replace it pre-failure, or to wash it with fresh water, if technicians could get to it soon enough. Newer equipment with contacts that were still coated with terminal grease did fine.
Application
Do you apply the anti-corrosion treatment to every connection and every crimp, or just certain types of connections? In our opinion, all connections in potential damp areas should be treated. In panel installations, we would minimize the coating.
Large glops give little benefit, and additional combustible material is never a good idea. It is possible under the correct circumstances of heat for grease to generate combustible fumes. Thus, the use should be moderate, and such use is approved.
For crimp connectors, apply a small amount to the wire after crimping; it serves no purpose in the crimp. The joint can then be heat sealed if desired, a preferred practice. The bolting surfaces should be lightly coated, and, if the location is particularly damp, a thin coating over the outside of the entire connection is advisable; we saw visual evidence that this helped. Even heat-sealed connections can be vulnerable if the sealing fails or if there are galvanic currents that attack the ring terminal itself and remove the tin coating. A more generous coating did seem to help the aluminum installations, though it was not a cure-all.
Because of the nature of this report, all recommendations are provisional. Look for an update on this project in six months.
Because connections develop high resistance under load before they fail, continuity testing was done with a 10-amp lighting load (except for samples that failed, which would not pass current). Power was supplied by the house bank of a boat plugged into a shorepower charger. A multimeter capable of measuring a 10-amp current was placed in series with the samples and a 100-watt nominal 12-volt deck light. The tinned copper terminal strips used are isolated between adjacent terminals. We have not used ground terminals or other types in which neighboring samples are connected. However, because of the salt film used in the test chamber, it is certain that galvanic currents between samples exist and have affected the results. The effect should have been both realistic and manageable, as we did isolate the aluminum samples on a separate strip.
We are testing a limited number of tin-tin spade connections, both with and without corrosion preventatives. These can be found on many sump pumps and water pumps.