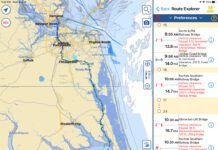
Chances are good that anyone who owns or has worked on a vessel equipped with an iron keel has at one point or another come across products that, “turn rust back into sound metal,” or make other similar and often impossible-sounding claims. The question is: Do they work, and is this the best approach for an iron keel? 288 Make no mistake about it, iron is cheap, and thats the primary reason for its use where keels and centerboards are concerned. Lead is nearly always preferred, at least for ballast keels, because its denser and weighs more for a given volume than iron, and it doesn’t corrode. Some vessels use bronze as a centerboard material, and while its not as dense as iron, all sailors know it does not corrode. Irons use as a ballast keel or centerboard material comes at a price, a price that is paid over the life of the vessel by its owner or owners. If the iron is not properly isolated from the water in which the vessel floats, it rusts prodigiously. Some studies have shown that an inch of iron will create 16 inches of shale rust. The insidious nature of the way in which iron rusts is often its, or the vessel owners, undoing. Unlike steel, which tends to slough off as it rusts, iron often retains its shape, rusting from the inside out. Thus, it may look sound, however, a sharp blow from a hammer may release huge chunks of material. This type of decay is referred to as graphitization because graphite residue is all that remains. So, can brush-on cures solve this problem? In our experience the answer is a qualified “No.” If the metal is a mass of shale thats falling to pieces, then no liquid is going to correct, halt, or reverse this problem. De-shaling, sandblasting, and then coating with a marine epoxy two-part primer is the only permanent method of arresting, but not reversing, this scenario. 
Some brush-on products weve used, however, do appear to arrest mild cases of rust on iron surfaces. One, Ospho (www.ospho.com), claims that it causes, “iron oxide (rust) to chemically change to iron phosphate-an inert, hard surface substrate that turns metal black.” Weve used this product. Provided no attempt is made to apply it over loose shale, it does stabilize the oxidation process, thereby halting the rust. It is designed as a primer of sorts; other coatings must be applied over it, the thicker the better, in order to isolate the iron from future contact with water. Look for a future Practical Sailor review of these products and their effectiveness.